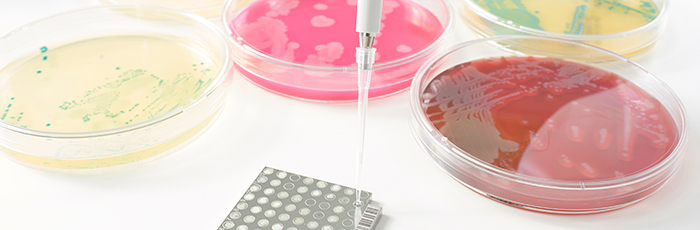
Disinfectant testing
Disinfectant testing is key to the production of safe and wholesome foods. We provide advice and testing of disinfectants and cleaning materials.
You may also be interested in
Transcript
Disinfectants should reduce levels of pathogens so that they do not cause disease, and reduce spoilage organism to minimise product contamination.
There are specific standards for laboratory testing of disinfection claims – such as the EN methods used in Europe. The method chosen depends on where and how the disinfectants are used and the specific target micro-organisms. All are tested in a 3 phase process - with each successive phase simulating in-use conditions more closely.
Phase 1 tests the formulation's active ingredients individually. Each ingredient is diluted in sterile distilled water; micro-organisms are added, and the reduction in microbial count in a given time is calculated.
Phase 2 testing is on disinfectant formulations as sold. Phase 2 Step 1 tests use aqueous suspensions, a wider range of test organisms and an interfering substance to simulate typical dirt the formulation will encounter.
Phase 2 step 2 tests are carrier tests in which micro–organisms with interfering substance are dried on to stainless steel discs, and the disinfectant formulations then tested.
In both steps 1 and 2, the reduction in microbial count in a given time is determined.
Phase 3 tests are field trials.
These validate the disinfectant in real life situations. They are normally performed over a number of weeks. The number of micro–organisms present on surfaces can be determined before cleaning, after cleaning and after disinfection.
Results can be compared to ascertain whether there is a reduction at each stage of the clean, and to compare the effects of the trial disinfectant with an existing product.