Food microbiology

To discuss your needs
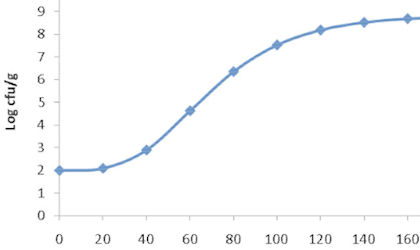
Microbiological food spoilage and food poisoning are two of the major concerns of most food companies. Preventing food poisoning and reducing or delaying spoilage are priorities for all manufacturers and retailers. At Campden BRI, we offer microbiology testing. As well as routine microbiological analysis to determine levels of microorganisms in foods, we can identify what the organisms are, and whether they will affect the shelf-life of the food, as well as investigating the source of any contamination problems. We can evaluate real factory scenarios in our newly commissioned Microbiology Process Hall, and can also offer advice if you have a problem with virus contamination.
We also have expertise in the response of microorganisms to food processing regimes (e.g. washing, heating, high pressure), and can advise on how changes to ingredients, packaging and processing as part of product development might affect microbial shelf-life - identifying the hazards and risks of each situation.
For those companies with in-house microbiological, and chemistry, testing facilities, we can offer advice on which methods to use, as well as on laboratory design, with laboratory approval/certification available through CLAS, the Campden Laboratory Approval Scheme. And our relaunched Campden Microbiology Proficiency Scheme will help you assess how good your laboratory analyses are.
Does your laboratory undertake pathogen tests for M&S plc and/or Tesco plc? It will need to participate in the Campden BRI Retailer supplementary audit (RSA) scheme to meet these retailers' requirements
You may also be interested in:
- Predictive microbiological models – what are they and how can they be used in the food industry | White paper
- Sous vide is the cooking of a vacuum–packed product at a low temperature in a water bath. This video demonstrates a safe sous vide cooking method | Video
Key services
COVID-19 (SARS-CoV-2)
Environmental testing and prevention to help the food industry control SARS-CoV-2.

Microbiological analysis and testing
Covering pathogens, spoilage organisms, and indicator organisms

Microbiological shelf-life
Product safety, retaining sensory, microbiological and chemical characteristics.

Microbiological methods evaluation
Evaluating microbiological methods, validation and interpretation.

Heat resistance
Determine whether the process you are using will be sufficient to achieve your aims.
Detection and control of foodborne viruses
Foodborne viruses are a safety challenge for a range of foods.
Microbiology training courses
Explore our microbiology related courses including; Understanding microbiology for non microbiologists and Setting shelf life: how to do it better
Are you getting the most from your Membership?
Watch our membership FAQ videos and find out more about Member Service Account spending, Member Interest Groups, help and advice
Where we refer to UKAS Accreditation
The Campden BRI group companies listed below are accredited in accordance with the recognised International Standard ISO17025:2017 by the United Kingdom Accreditation Service (UKAS). The accreditation demonstrates technical competence for a defined scope of methods, specific to each site, as detailed in the schedules of accreditation bearing the testing laboratory number. The schedules may be revised from time to time and reissued by UKAS. The most recent issue of the schedules are available from the UKAS website www.ukas.com. Campden BRI (Chipping Campden) Limited is a UKAS accredited testing laboratory No. 1079