Microbiological methods evaluation

To discuss your needs
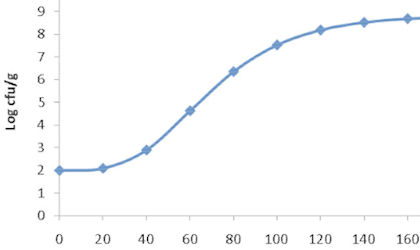
Microbiological methods can be complex. They don't always give exact answers, and understanding what results mean is of great commercial importance when deciding on a manufacturing course of action. It is vital that appropriate methods are used and that they are shown to be suitable for a particular situation. Campden BRI experts can help you to reach the right decisions.
We provide a wide range of services for evaluating microbiological methods, validation and interpretation. Validation can be to your company's own standard, or to one of several officially recognised standards. Don't use inappropriate methods, or draw the wrong conclusions from the right result!
Key services
COVID-19 (SARS-CoV-2)
Environmental testing and prevention to help the food industry control SARS-CoV-2.

Microbiological analysis and testing
Covering pathogens, spoilage organisms, and indicator organisms

Microbiological shelf-life
Product safety, retaining sensory, microbiological and chemical characteristics.

Microbiological methods evaluation
Evaluating microbiological methods, validation and interpretation.

Heat resistance
Determine whether the process you are using will be sufficient to achieve your aims.
Detection and control of foodborne viruses
Foodborne viruses are a safety challenge for a range of foods.
Microbiology training courses
Explore our microbiology related courses including; Understanding microbiology for non microbiologists and setting shelf life: how to do it better
Are you getting the most from your Membership?
Watch our membership FAQ videos and find out more about Member Service Account spending, Member Interest Groups, help and advice
Where we refer to UKAS Accreditation
The Campden BRI group companies listed below are accredited in accordance with the recognised International Standard ISO/IEC 17025:2017 by the United Kingdom Accreditation Service (UKAS). The accreditation demonstrates technical competence for a defined scope of methods, specific to each site, as detailed in the schedules of accreditation bearing the testing laboratory number. The schedules may be revised from time to time and reissued by UKAS. The most recent issue of the schedules are available from the UKAS website www.ukas.com. Campden BRI (Chipping Campden) Limited is a UKAS accredited testing laboratory No. 1079