Accelerated shelf-life testing

To discuss your needs
We understand that when a product launch deadline is tight, it is not always practicable to carry out real time storage trials – especially for products with a long shelf-life.
Applicable products
Accelerated shelf-life testing should not be used to determine microbiological safe shelf-life of perishable foods with a ‘Use by’ date. Read why in our accelerated shelf-life testing blog.
Accelerated shelf-life testing can however be applied, to varying extents, to a wide range of ambient products, and dry products. It may also be applied to frozen products if you tell us what storage conditions you want us to use. Get in touch to see what we can do for your product.
We can help to find the right conditions for your product
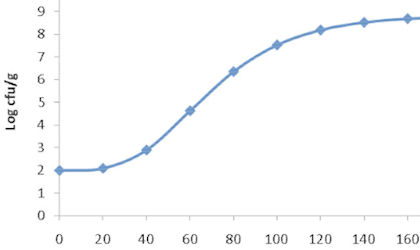
Increased storage temperature is generally used as the accelerating storage condition and there are mathematical models we can use to link temperature to the rate of product deterioration.
Other abuse conditions include increasing, for example humidity, oxygen, and/or light exposure. We can help to find the conditions that are most applicable for your product. Or we can simply apply the conditions specified by you – taking the storage and testing out of your hands, so that your resources can be focused elsewhere.
Getting the most out of accelerated shelf-life testing
Where you do not already have an established accelerated shelf-life testing protocol, we can help you develop one for you product (by conducting testing under real time and accelerated conditions and modelling the comparisons).
To get the most useful data out of accelerated shelf-life testing, we recommend running these tests in parallel with real-time storage conditions, or testing a new product alongside a similar ‘control’ product that has already undergone real-time testing.
Key services
COVID-19 (SARS-CoV-2)
Environmental testing and prevention to help the food industry control SARS-CoV-2.

Microbiological analysis and testing
Covering pathogens, spoilage organisms, and indicator organisms

Microbiological shelf-life
Product safety, retaining sensory, microbiological and chemical characteristics.

Microbiological methods evaluation
Evaluating microbiological methods, validation and interpretation.

Heat resistance
Determine whether the process you are using will be sufficient to achieve your aims.
Detection and control of foodborne viruses
Foodborne viruses are a safety challenge for a range of foods.
Microbiology training courses
Explore our microbiology related courses including; Understanding microbiology for non microbiologists and Setting shelf life: how to do it better
Are you getting the most from your Membership?
Watch our membership FAQ videos and find out more about Member Service Account spending, Member Interest Groups, help and advice
Where we refer to UKAS Accreditation
The Campden BRI group companies listed below are accredited in accordance with the recognised International Standard ISO17025:2017 by the United Kingdom Accreditation Service (UKAS). The accreditation demonstrates technical competence for a defined scope of methods, specific to each site, as detailed in the schedules of accreditation bearing the testing laboratory number. The schedules may be revised from time to time and reissued by UKAS. The most recent issue of the schedules are available from the UKAS website www.ukas.com. Campden BRI (Chipping Campden) Limited is a UKAS accredited testing laboratory No. 1079