How to determine product microbiological shelf–life
6 February 2011 | Gail Betts, Head of Microbiology
Introduction
The length of time for which any particular food can be kept will depend on the nature of the food itself, and the preservation treatments to which it has been subjected. The type of packaging used to contain the food will also have an inherent effect. It is up to the manufacturer of the food to determine and assign the shelf life of the food they produce, keeping in mind the requirements of relevant legislation, such as the Food Safety Act 1990 and Regulation (EC) No 178/2002 (the ‘General Food Law Regulation’). These measures, in essence, require that a food must be both of the nature, substance and quality expected by the consumer, and also not injurious to health for any reason. Thus, it is illegal to sell food which has deteriorated during storage to be unfit for health reasons, or if its quality has deteriorated beyond that which would be normally acceptable. Food manufacturers go to great lengths to ensure that this does not happen.
The assignment of shelf-life is very important. If it is too short then manufacturing costs may be high and profit margins low. Also, there might be significant wastage of perfectly good product, disposed of in the belief that it is no longer fit for consumption. If it is too long then there is the potential for food quality to become poor or unacceptable, or for the growth of food pathogens to occur - and the product will not meet the requirements of food safety legislation. It is therefore important to assign the shelf-life in a systematic and scientific manner, taking all relevant factors into consideration. Evaluation of shelf life is also important when products are reformulated; for example, minor changes inproduct reformulation can have a major impact on growth of microorganisms, or on product texture and stability. Any product formulation change should trigger a re-evaluation of the product's shelf life.
Download the complete Maximising Shelf-life eBook, for free, to unlock the tools for measuring and maximising the shelf-life of your food and drink products whilst ensuring their safety and quality.

Factors affecting shelf life
The shelf-life of a food product is influenced by many aspects of manufacturing, product formulation and storage conditions; the effect of these on the growth of target microorganisms must be considered.
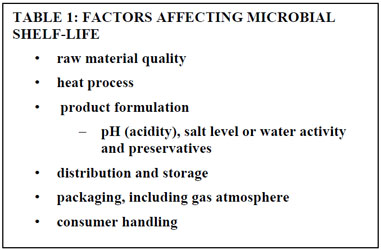
Table 1: Factors affecting microbial shelf-life.
The food processor or manufacturer should have enough knowledge of his product to be able to determine what factor is going to limit the product's shelf-life, and the approximate time for which the product will remain fit for eating (i.e. days, months or years). This will include comparison with similar products and a clear insight into the differences between these 'similar' products and the product in question. When microbiological issues are important, there are three different basic approaches that are used to assess product shelf-life: shelf-life trials, challenge tests and predictive microbiology. Each has a role to play in assuring the safety of the product shelf-life chosen.
Methods for assessing shelf life
Shelf-life trials
This is designed to answer the question: for how long does this product remain within the designated quality parameters during normal production and storage conditions? Such tests only assess the growth of micro-organisms naturally present in the batch of product tested. As it is unlikely that pathogens would be present in product then shelf-life testing will not be able to assess the potential for growth of foodborne pathogens.
The shelf-life of food products is determined in a logical sequence of events:
(i) Kitchen/pilot scale assessment where the product and process characteristics are defined and a target shelf-life decided.
(ii) Factory scale trials, where the majority of laboratory testing is done on batches of product produced under routine manufacturing conditions and where the shelf-life of the product is assigned. During this stage, product is stored under conditions to which it is likely to be exposed during retail distribution and examined for any changes in levels of target microorganisms. Storage of samples should be done using a recognised protocol as shown in Table 2 for chilled food products
(iii) Full scale production, where any changes to the shelf-life are monitored.
It should be noted that assigned shelf-life determined in these studies is only relevant to the product formulation and storage conditions used and cannot be extrapolated to other conditions. One limitation with shelf-life evaluation trials is the assumption that the consumer will follow the storage instructions given on the package. Allowance should be made for a reasonable degree of product abuse.
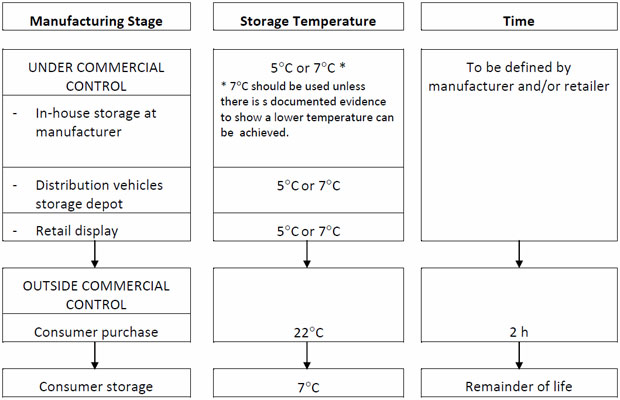
Table 2: Recommended storage regime for shelf-life trials and challenge tests for chilled foods (Betts et al 2004).
Challenge testing
This is designed to answer the question: Will the product formulation and storage conditions control growth of pathogens (or spoilage organisms) during the designated shelf-life if they were present in the ingredients or contaminated the food during manufacture?
It involves the deliberate inoculation of a food with the relevant organisms followed by growth studies under controlled laboratory conditions. The advantage of this technique is that it provides data to answer the ‘what if’ questions that may not be answered during shelf-life trials . For example:
- what would happen if Listeria monocytogenes contaminated my product after cooking?
- what would happen if a preservative-resistant yeast survived the processing?
Obviously, in some foods, experience and/or mathematical models (see below) will show that microbial growth is not possible, and that microbial challenge testing is unlikely to yield any useful information. It is important to use existing knowledge and mathematical growth models, if appropriate, in order to ‘home in’ on areas of potential concern.
In other situations, the likelihood of the presence of pathogens in the product after processing may be so small as to make challenge testing of no practical value. It may be possible to demonstrate that a pathogen can grow in a product, but the challenge test does not indicate the likelihood of it being there. These tests need to be carefully planned and expert knowledge is required for the interpretation of results.
Challenge tests are of most value when the organisms used represent those that might realistically be present in the product, either from the ingredients or via post-process contamination. The levels added in the tests also need to be realistic – adding large numbers of Listeria, say, when contamination levels, if they occur, are almost certain to be low, is of limited value.
Challenge tests will not give an estimation of shelf life, but they will indicate whether the shelf-life proposed is inherently safe.
Predictive microbiology
This involves the use of mathematical models to predict the likely growth of spoilage organisms or food pathogens in different product formulations or storage conditions. It provides a rapid answer for use in new product development and trouble shooting situations. Data can be obtained on the length of lag time, rate of growth, time taken to reach a target number of organisms, and effect of different storage regimes. In addition, many models can be used to predict the effect of fluctuating temperature profiles or changes in pH as may be seen during manufacture of fermented foods.
The development of these models is not straightforward. Individual microorganisms, even different strains within the same species, have very different growth characteristics, and this growth will be affected by:
- the food matrix itself (e.g. growth of a certain organism in pureed vegetables may be very different from that in meat or fish)
- the general availability of nutrients and the physiological state of the organism itself
- the number of organisms initially present
- the likely competition for nutrients from other organisms.
Despite this, significant progress has been made. More information can be found in Campden BRI Key Topic No. 9: Food preservation: an introduction.
Predictions for foodborne pathogens can be found at www.combase.cc
Predictions for a range of spoilage organisms are shown in Table 3. View a comprehensive description of predictive microbiology.
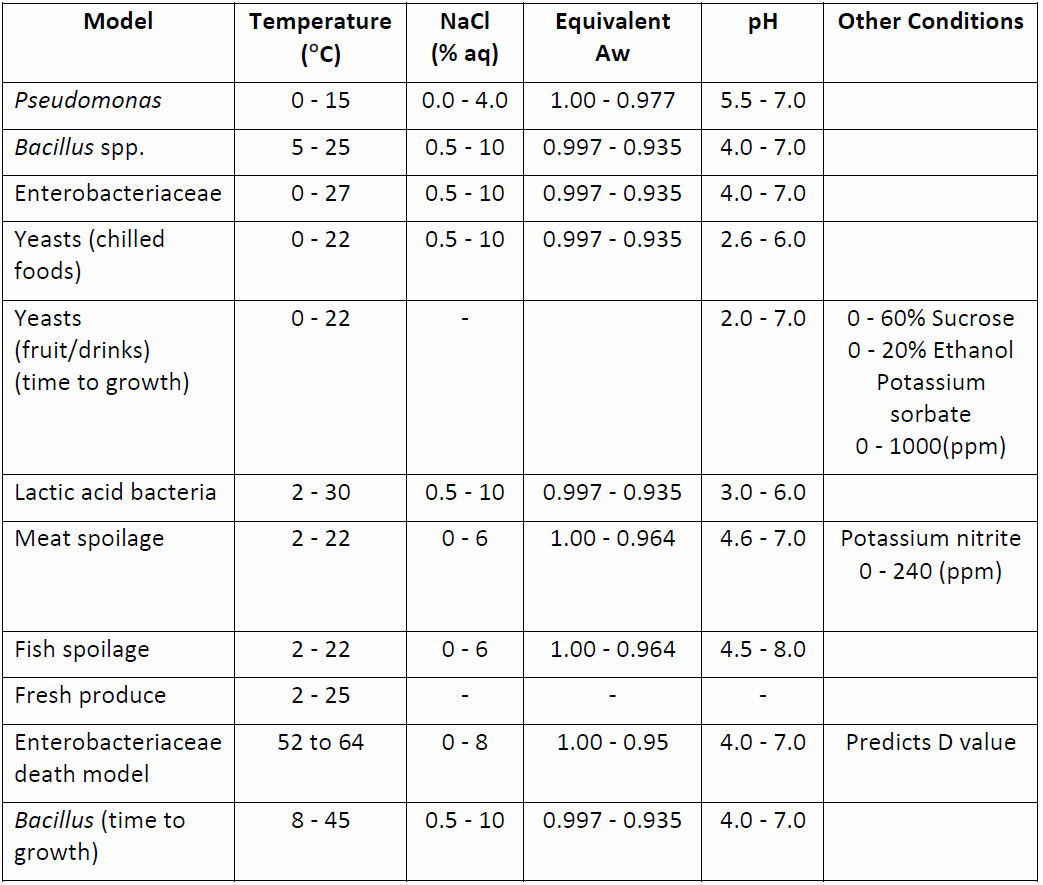
Table 3: Predictive models for spoilage organisms in Campden BRI Forecast system.
Case studies
The approach taken with shelf-life testing is dependent on the type of product being assessed. The pH, Aw, heat process and storage temperatures will select for different target organisms and different end point criteria. Examples of the approaches used are given below for two different food groups.
Shelf-life of ambient stable acidified foods
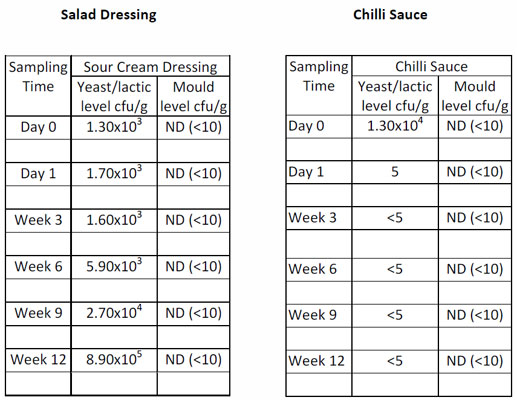
This procedure is used by many manufacturers of acidified foods. Products are inoculated with a cocktail of acid tolerant yeasts, moulds and lactic acid bacteria. If the levels increase during the trial then the product is considered to be unstable (CIMSCEE, 1992). However, if the levels decrease then the products are considered to be stable for the duration of the ambient shelf-life (typically 18-24 months).
Two sets of data are shown below for different product types. It can be seen that the salad dressing has allowed growth of the target organisms. It is therefore not stable and needs to be reformulated whilst the chilli sauce formulation has shown a rapid decrease in levels of microorganisms and is likely to be stable throughout the desired shelf-life.
Shelf-life of a chilled, cooked meat product
The shelf-life of this product type is short (typically 2-4 weeks) and is usually governed by levels of general spoilage microorganisms. Samples of product are stored according to the recommended storage regime and examined for levels of total viable count (TVC) and levels of indicator organisms such as Enterobacteriaceae (IFST, 1999). The levels are compared to published guidance in order to assign an appropriate shelf-life. For sliced meat products, the guidance recommends a maximum level of 107 cfu/g for TVC and 104 cfu/g for Entoerobacteriaceae. These levels are reached rapidly (Figure 1) and therefore the shelf life of this product would be restricted to 4 or 5 days. The counts are presented as log10 colony forming units (cfu) per gram.
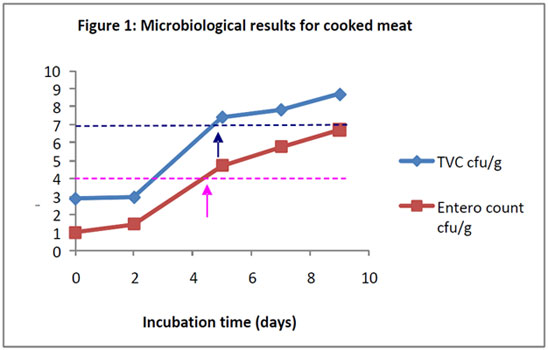
Figure 1: Microbiological results for cooked meat.
Meeting legislative requirements
There are two important areas in which challenge testing or predictive models can be used to help assess the safety of the designated shelf-life of chilled food products. These are (i) growth of Clostridium botulinum and (ii) growth of Listeria monocytogenes in chilled foods.
For many years it has been recommended that chilled modified atmosphere packaged or vacuum packaged foods have a restricted shelf-life of up to 10 days unless they meet one of the recommended controlling factors required to inhibit the growth of C. botulinum (Betts, 2009)
- Heat treatment of 90C for 10 minutes (or equivalent)
- Aqueous salt level of 3.5% or more
- Water activity of 0.97 or less
- pH of 5.0 or less
If none of these factors are achieved in a food product, the safety of other levels of these factors or alternative preservatives can be demonstrated by inoculated challenge test or predictive models.
With respect to L. monocytogenes, chilled ready-to-eat products (RTE), other than those intended for infants or for special medical purposes, should have no more than 100 cfu/g L. monocytogenes present at the end of shelf-life (EU Regulation EC 2073/2005, as amended). Food business operators producing RTE products able to support growth must therefore be able to demonstrate that the product will not exceed the limit of 100 cfu/g throughout its shelf-life. When this is not demonstrated, it must be shown that Listeria is absent (not detectable) in the product at the end of the manufacturing process.
Challenge testing or predictive models are two ways that manufacturers can demonstrate the safety of the assigned shelf-life with respect to the growth of L. monocytogenes in RTE foods following appropriate guidelines (Betts, Brown and Everis 2004; AFSSA, 2009).
Conclusions
The shelf-life of food products is influenced by microbiological, chemical and sensory considerations - along, in some cases, with legislative requirements. It needs to be determined following sound scientific principles that can take into account all the relevant formulation, manufacturing, distribution and storage factors. The shelf-life determined is unique to the product and storage conditions tested and cannot be extrapolated to other products or storage conditions. Assigning the correct shelf-life can be the key to the commercial success of a new product and should be done in the early stages of new product development.
White paper download
Click below to download a copy of the white paper in PDF format
How can we help you?
If you’d like to find out more about how we can help with the shelf-life of your products, contact our Support Team.
References
- AFSSA. (2008) Agence Francaise de Securité Sanitaire des Aliments (2008). Working Document. Version 2 - November 2008. Technical Guidance Document on shelf-life studies for Listeria monocytogenes in ready-to-eat foods.
- Betts, G.D. (2010) Challenge testing protocols for assessing the safety and quality of food and drink. Guideline No. 63, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD.
- Betts, G.D. and Betts, R.P. (2009) The manufacture of vacuum and modified atmosphere packaged chilled foods: A Code of Practice (Second Edition). Guideline No. 11, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD.
- Betts, G.D., Brown, H.M. and Everis, L.K. (2004) Evaluation of product shelf-life for chilled foods. Guideline No. 46, Campden BRI, Chipping Campden, Gloucestershire, GL55 6LD.
- CIMSCEE (1992) Code for the production of microbiologically safe and stable emulsified and non-emulsified sauces containing acetic acid (1992). Comité des Industries des Mayonnaises et Sauces Condimentaires de la Communaute Economique Européenne. Brussels.
- FSA (2008) Guidance on the safety and shelf-life of vacuum and modified atmosphere packaged chilled foods with respect to non-proteolytic Clostridium botulinum. www.food.gov.uk/foodindustry/guidancenotes/foodguid/vacpac
- IFST (1999) Development and use of microbiological criteria for foods. IFST Booklet. ISBN 0 905367 16 2.