Instil confidence in your air cleaning system through validation
The Virology Team at Campden BRI, a leading food science & research organisation, offer a range of tests to validate the efficacy of your systems to safeguard your customers and enhance your reputation as a manufacturer of quality air cleaning systems.
In the wake of Covid-19 driven lockdowns, employers want to ensure they are providing a safe working environment - safeguarding productivity and revenue, whilst limiting the spread of infection. They are looking to manufacturers of proven technologies to help achieve this level of safety. Campden BRI can support claims that your air cleaning systems and usage protocols meet desired levels in terms of inactivating a range of airborne viruses. Campden BRI have developed and validated a test in their purpose-built aerobiology microbiology laboratory, which uses a ‘surrogate’. Structurally similar to a target virus, it aim is to verify whether cleaning regimes inactivate or remove viruses in a real-to-life setting. Campden BRIs use of their bespoke safe-to-use ‘surrogates’ has allowed them to eliminate the biological safety challenges of working with active human pathogens. Indeed, it also allows them to test control measures in the real environment (for example, on a factory or retail surface) and even on hands. This test has been successfully applied to technologies based on plasma, UV irradiation, air filtration, ozone, hydrogen peroxide and disinfectant fogging.
Annette Sansom, Microbiology Lead at Campden BRI explained the surrogate story,
“Strictly speaking viruses aren’t ‘alive’ – not in the way that bacteria, plants and animals are alive. They are physical structures that hijack living things to make many copies of themselves – It is their physical structure that is beneficial. Other viruses with a similar structure will ‘behave’ in a similar way in many environmental circumstances. And these provide the surrogate approach. One of our surrogates is a bacteriophage called Phi6 (a virus that infects bacteria). It is structurally similar to SARs and influenza viruses, but doesn’t infect plants or animals, including humans, and so is perfect for any trials that we want to run inhouse or externally.”
Campden BRI are rightly proud of their innovative testing package and can test the efficacy of cleaning and disinfection methods by using a range of surrogates. In the case of Phi6, the test involves growing a lawn of Pseudomonas syringae in a petri dish and introducing it to a test environment, with a view to seeing if the virus is present and active. Any activity will be apparent from the appearance of holes (plaques) in the surface of the bacteria lawn, as Phi6 kills its host. If a cleaning chemical or disinfectant has inactivated the phage there will be fewer or no plaques in the lawn – so you can compare different cleaning systems based on how much they can prevent plaques compared to control samples.
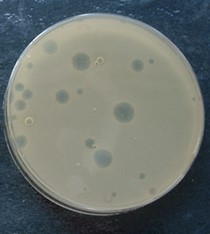
Figure 1. The circular ‘plaques’ are areas where bacteria in the pale lawn have been killed by phage
Campden BRI are keen to help you get your approach to sanitising and cleaning right for you and your environment. They have a long-established reputation for assessing the efficacy of cleaning chemicals for killing bacteria (bactericides) in suspension (following BS EN 1276) and on surfaces (following BS EN 13697). The cleaning service is just one of a range of services that Campden BRI offer to help you guard against airborne pathogens.
For more information on the Air Cleaning Validation Service or other virology services, please contact:

About Annette Sansom
Annette has a wealth of knowledge and experience from working at Campden BRI since 1998, always within Microbiology.
Annette’s food and drink industry interests are: microbiology including bacteria, viruses, fungi and protozoa; food safety; food spoilage; fresh produce microbiology including vertical farming and methods to describe microbial populations.

